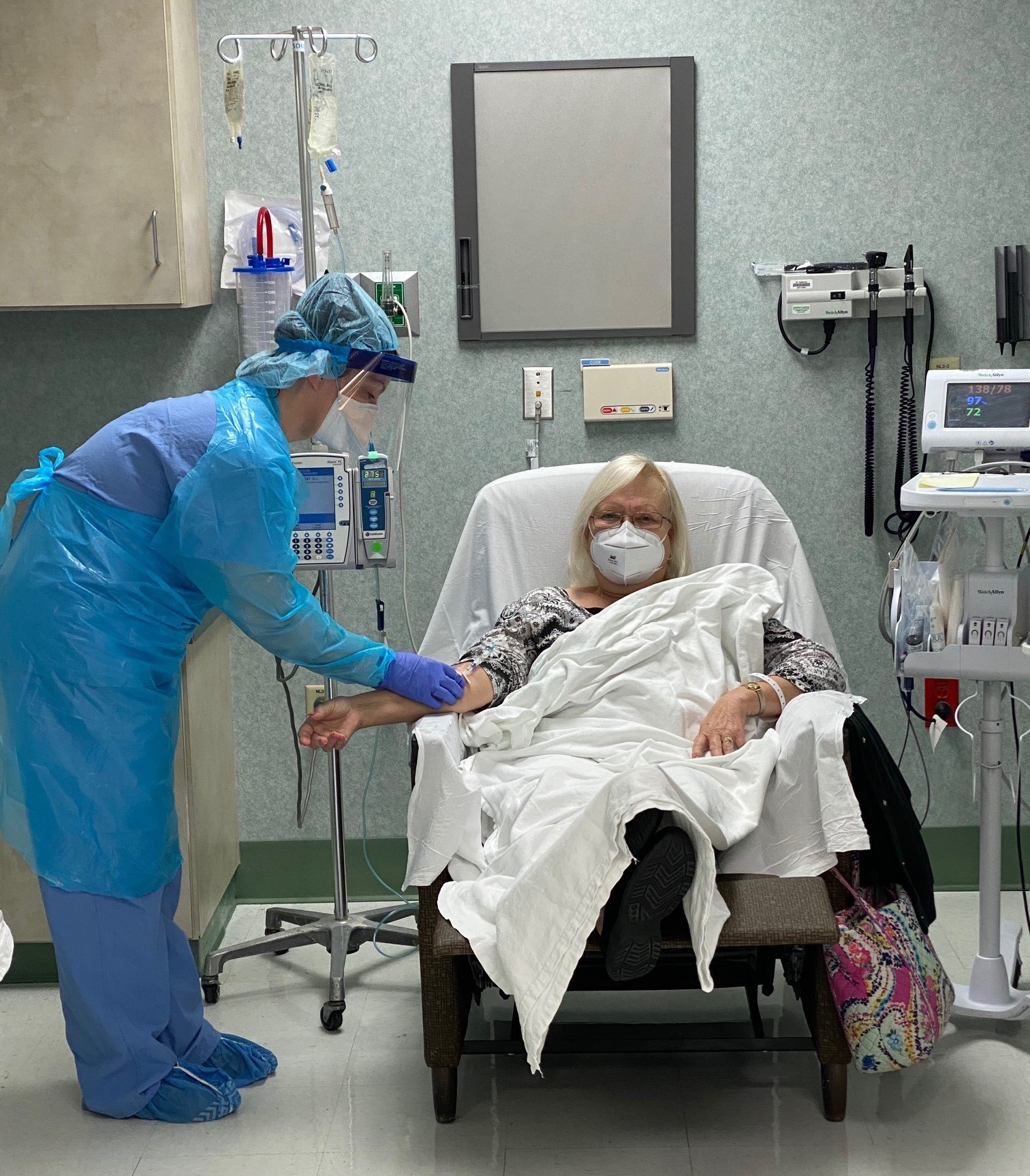
Fort Walton Beach Medical Center is now offering bamlanivimab antibody infusions to non-hospitalized patients who have tested positive for COVID-19 and have other high-risk factors. The first patient received the treatment on December 22.
According to a hospital spokeswoman, Fort Walton Beach Medical Center is the only hospital in the tri-county area offering this antibody infusion.
The U.S. Food & Drug Administration (FDA) granted Emergency Use Authorization (EUA) of bamlanivimab for the treatment of COVID-19 patients experiencing mild to moderate symptoms who are high risk to develop severe COVID-19. Fort Walton Beach Medical Center will provide the outpatient IV treatment for adults and adolescents age 12 and older.
High-risk conditions that may be eligible for this treatment include:
- Obesity
- > = 65 years of age
- Chronic kidney disease
- COPD
- Diabetes
- Immunosuppressive disease
- Heart disease
- For children over the age of 12, sickle cell disease, neurodevelopmental disorders, and asthma are also among several additional considerations for eligibility.
“We’re committed to providing our patients, caregivers, and the communities we serve with the latest treatments in the fight against COVID-19 and are excited to bring this option to the tri-county area,” said Dr. Bob Kiskaddon, Chief Medical Officer for Fort Walton Beach Medical Center. “We will support all of our local hospitals in working closely with physicians and other providers across the communities we serve to provide treatment to patients who may benefit, per FDA guidelines.”
The medication supply is limited and based on availability. Patients will need a physician referral and meet FDA criteria prior to scheduling. The treatment is a one-time intravenous infusion over the course of one hour followed by an hour of monitoring. Scheduling for patients who meet the criteria can be made by calling Emerald Coast Infectious Disease at 850-862-3979.
bamlanivimab is not authorized for use for patients who:
- Are hospitalized due to COVID-19
- Require oxygen therapy due to COVID-19
- Require supplemental oxygen for other conditions and require an increase in their oxygen requirement.
For more information on monoclonal antibody treatment for COVID-19, visit the Coronavirus Hub at www.fwbmc.com/covid-19.